Disulfide bonds are essential components in the three-dimensional structure of many proteins. These covalent bonds can be found in almost all extracellular peptides and proteins. A disulfide bond is formed when the sulfur atom of one cysteine forms a single covalent bond with the sulfur atom of the other cysteine at a different location in the protein. These bonds help stabilize proteins, especially those secreted from cells.
If the polypeptide contains only one pair of Cys, the formation of the disulfide bond is simple. The peptides were synthesized in solid or liquid phase and then oxidized in a solution of pH8-9.
When it is necessary to form two or more pairs of disulfide bonds, the synthesis process is relatively complex. Although the formation of disulfide bonds is usually completed in the final stages of the synthesis protocol, sometimes the introduction of preformed disulfides is advantageous for joining or lengthening the peptide chain. Sulfhydryl groups are usually used as protective groups such as trt, Acm, Mmt, tBu, Bzl, Mob, Tmob and so on. We have listed two routes for the formation of polydisulfide bonds on peptides synthesized with 2-Cl resin and Rink resin as carriers respectively:
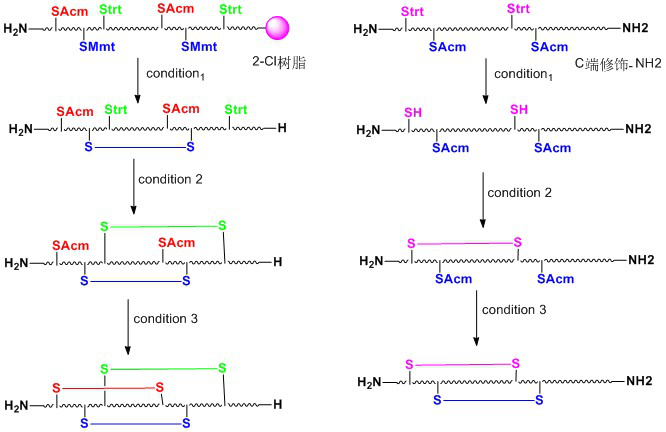
Polypeptide ring by disulfide bond
Monodisulfide polypeptide,
Didisulfide polypeptide,
Triple disulfide polypeptide,
Intermolecular symmetric disulfide polypeptide,
Intermolecular asymmetric disulfide polypeptide,
Polypeptides formed disulfide bonds by mercapto-Mpa mercaptopropionic acid.
Ordering method
1) Send the peptide sequence to be synthesized to the mailbox: , after receiving the mail to determine whether it can be synthesized, but also can contact our sales staff.
2) The peptide synthesis contract and confidentiality agreement are signed, and payment is made after use.
3) According to the contents of the contract, delivery within the specified time and provide an invoice